Being infected with COVID-19 puts a person at equal or much higher risk of adverse events than COVID-19 vaccination, for 179 conditions tested

We compare the risk of experiencing an adverse event (from a defined list of 179 medical conditions), either following COVID-19 vaccination or when infected with COVID-19 disease. We show that contracting COVID-19 carries the highest risk by far, of experiencing adverse conditions.
Publication date: 3 November 2021
Authors:
Shirley Collie (Chief Healthcare Analytics Actuary, Discovery Health), Lizelle Steenkamp (Head of Risk Intelligence, Discovery Health), Jared Champion (Senior Actuary, Discovery Health), and Tommy Chen (Actuary, Discovery Health)
This analysis considers claims data (claims submitted by members of medical schemes administered by Discovery Health). This data is used to:
1) Analyse rates of one of 179 serious adverse events - post-dose one of the Johnson & Johnson (J&J) COVID-19 vaccine or post each dose of the two-dose Pfizer vaccine.
2) Compare the relative risk of experiencing an adverse event from either COVID-19 vaccination (with the J&J or Pfizer COVID-19 vaccine) versus from a COVID-19 infection (SARS-CoV-2 infection).
- Mild vaccine-related adverse events such as fever, malaise, and local injection-site reactions were not included in our analysis.
Overcoming vaccine hesitancy and fighting misinformation is key to achieving vaccination targets
South Africa has procured sufficient COVID-19 vaccine doses for around 80% of the population. Furthermore, the National Department of Health has set targets to see 70% to 80% of the adult population vaccinated by December 2021. However, based on current vaccinated population levels and projections, it is unlikely that South African will meet these targets.
The pace of vaccination is dictated by several factors. However, there is growing consensus that COVID-19 vaccine hesitancy is a key driver of the relatively slow pace of vaccination.
Vaccine hesitancy is fuelled in large part, by confusion over the degree to which people might experience side-effects post-vaccination - both common, mild side effects and rarer, but very serious, adverse events. Large-scale misinformation and fake news around vaccine side-effects (particularly around the risk and rate of serious side effects) adds to the confusion people feel.
Another driver of vaccine hesitancy is a belief that people are more likely to experience severe adverse events from COVID-19 vaccination than from COVID-19 illness itself. Our analysis shows that this is not the case. In fact, contracting COVID-19 illness comes with far a higher risk of severe outcomes than COVID-19 vaccination.
By 2 November 2021, almost 22.5 million COVID-19 vaccine doses had been administered in South Africa.
Of these, 2.5 million had been administered to members of schemes administered by Discovery Health, most of whom were vaccinated from 17 May 2021 onward - when the country's mass vaccination programme kicked off.
The extent of vaccine penetration within the Discovery Health client base to date, allows us to analyse the risk of vaccine-related adverse events in this population.
Methodology
First, we defined a clinically relevant set of potential vaccine-related adverse events made up of 179 conditions. Among these 179 conditions, were 25 medical conditions considered in Barda, 20211.
Overall, the 179 adverse events we selected were based on the following criteria:
- Adverse events identified from clinical trials (e.g., lymphadenopathy and Bell's palsy) - as per Barda, 20211.
- Potential adverse events identified by Discovery Health as being immune-mediated. This process resulted in a further 154 conditions being added to the list (over and above those considered in Barda, 2021) bringing the list to 179 conditions examined.
We then investigated the presence of these 179 potential adverse events in the members of schemes administered by Discovery Health who had received either the J&J COVID-19 vaccine or the Pfizer COVID-19 vaccine from 17 February 2021 to 28 September 2021. The presence of these conditions was assessed based on a healthcare claim to any provider.
This analysis includes only adult medical scheme members. We allowed for a 42-day follow-up period after either the first or second vaccine dose.
1) First, for each potential adverse event, we identified a population of vaccinated members who had no previous diagnosis of that event (condition) and no documented prior COVID-19 illness (SARS-CoV-2 infection). These vaccinated members were then individually matched to unvaccinated members, or clinical twins, with the same:
- Age
- Sex
- Residential district
- Number of risk factors defined by the US Centers for Disease Control and Prevention (US CDC) as putting people at high risk of serious COVID-19 illness2
- Number of prior flu vaccinations (a proxy for the likelihood that a member will receive a scheduled vaccine, and engage in healthy behaviour)
- Medical scheme plan option (as a proxy for socio-economic status).
- We excluded scheme members who had a prior COVID-19 infection.
- We excluded scheme members who had the condition in question, historically.
2) Secondly, to allow us to compare the risk of an adverse event from vaccination to the risk of the same event from COVID-19 disease, we performed a similar analysis. We compared scheme members who had COVID-19 (SARS-CoV-2 infected) to scheme members who did not have COVID-19. Here, our total sample size was just under 180 000 people.
- We excluded scheme members who had been vaccinated prior to COVID-19 infection.
- We excluded scheme members who had the condition adverse event/condition in question historically.
Once we had the results of the above two analyses, we could compare the experience of the same set of adverse events in people who had been vaccinated and in people who had COVID-19 (SARS-CoV-2 infected).
Our results
Figures 1 to 3 show the relative risk (comparing risk in two groups) of adverse events in recipients of each type of COVID-19 vaccine, compared to the risk of the same events when infected with COVID-19.
The main insights from these figures are as follows:
Figure 1:
In the case of the J&J COVID-19 vaccine:
- The only conditions for which there is a statistically significant increased risk are:
- Paraesthesia with a 3.58-fold increased risk of an event (a feeling of burning, prickling, tingling or numbness usually felt in the hands and arms or legs and feet, but which can be felt elsewhere). This means people are four times more likely to experience this condition when they have the J&J vaccine than when unvaccinated.
- Myositis with a 1.9-fold increased risk of an event (inflammation of the muscles that you use to move your body). This means people are almost two times more likely to experience this condition when they have the J&J vaccine than when unvaccinated.
- All other conditions do not show a statistically significant heightened risk.
However, in the case of COVID-19 infection:
- There is a 39.6-fold increased risk of pulmonary embolism. This means that people are forty times more likely to experience this condition when they have COVID-19 than when they are not SARS-CoV-2 infected.
- There is a 22.4-fold increased risk of myocarditis.
- There is a 15.78-fold increased risk of acute kidney injury.
(There's no statistically increased risk of these three conditions following COVID-19 vaccination).
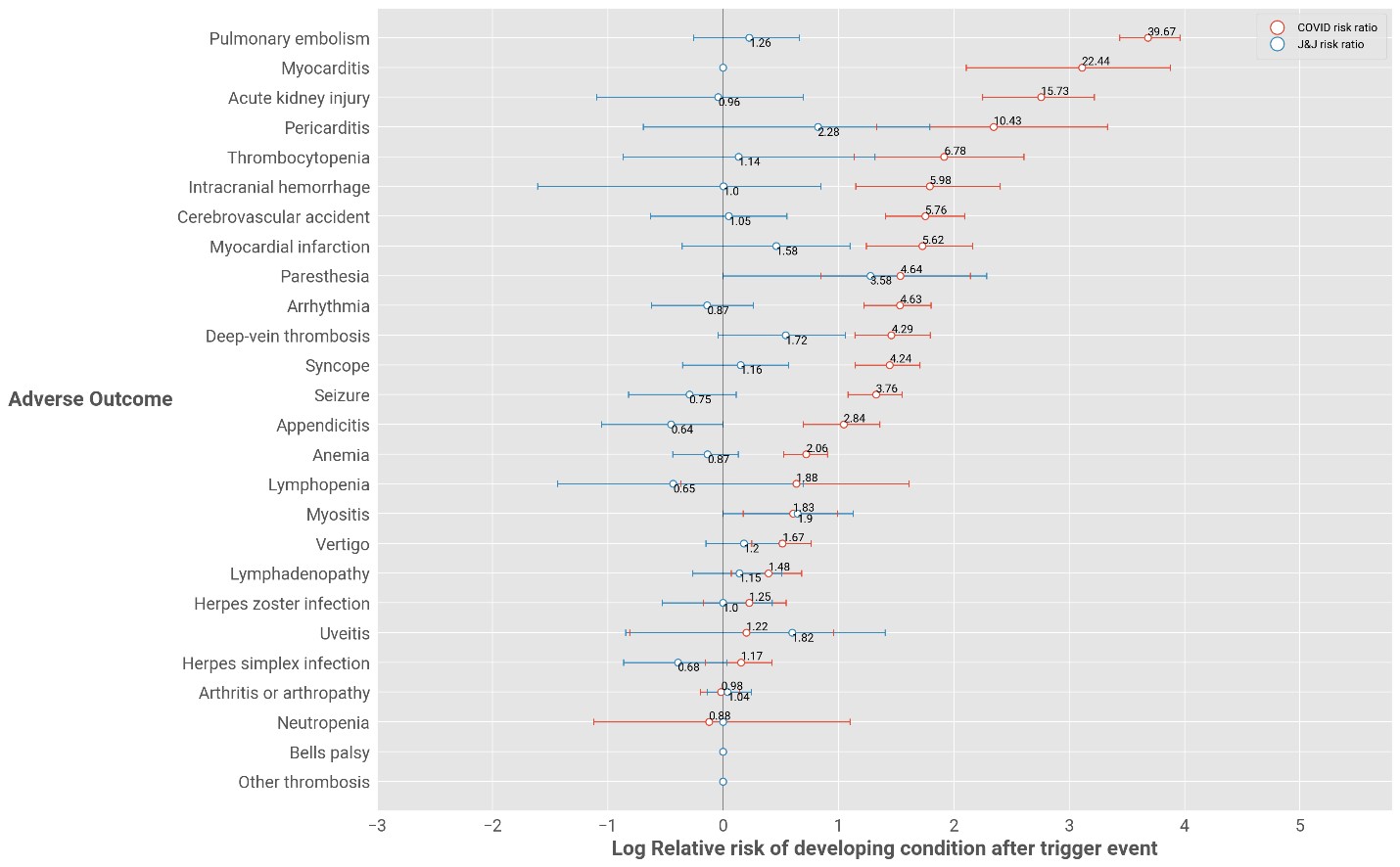
Figure 1: Relative risk of adverse events for J&J-vaccinated individuals versus individuals who have COVID-19
Figure 2
In the case of the Pfizer COVID-19 vaccine, post-dose one:
- The only condition for which there is a statistically significant increased risk is:
- Lymphadenopathy with a 1.65-fold increased risk of an event. (This is a condition or disease affecting the lymph glands resulting in lymph nodes that are abnormal in either size, consistency, or number)
- The risk of lymphadenopathy is 1.48-fold for COVID-19 infected individuals
In the case of COVID-19 disease, the top three conditions presenting risk are the same as those stated above and explained for in Figure 1. Also, there's no statistically increased risk of these three conditions following Pfizer COVID-19 vaccination, post-dose one - as shown in Figure 2.

Figure 2: Relative risk of adverse events for Pfizer-vaccinated individuals after dose 1 versus individuals who have COVID-19
Figure 3
In the case of the Pfizer COVID-19 vaccine, post-dose two there is no statistically significant increase in risk for any conditions.
The risks of adverse events relating to COVID-19 illness (mentioned in Figures 1 and Figure 2) remain the same.
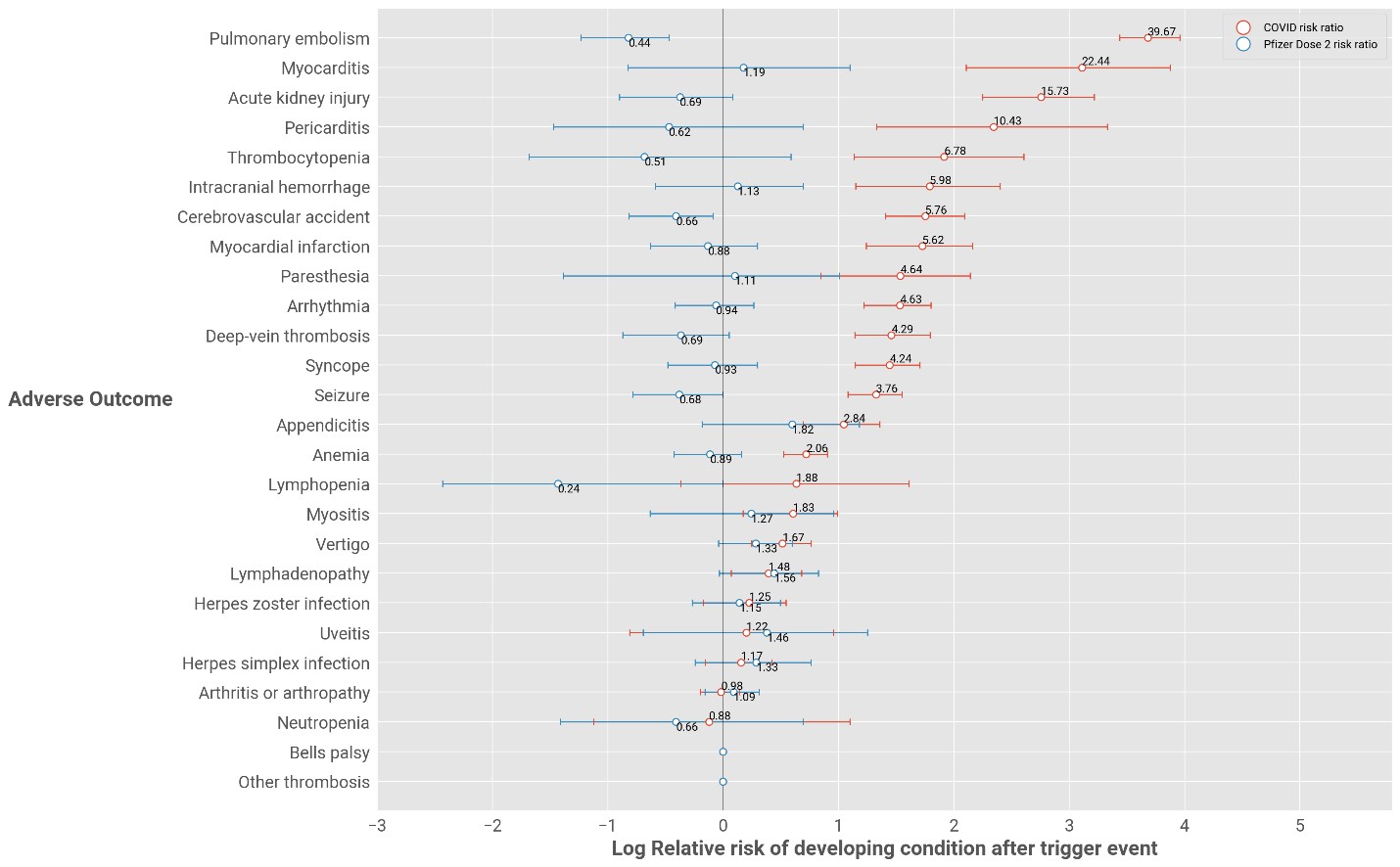
Figure 3: Relative risk of adverse events for Pfizer-vaccinated individuals after dose 2 versus individuals who have COVID-19
The absolute risk of a person experiencing one of nine serious conditions because of either COVID-19 illness or vaccination
While the figures 1 to 3, posted above, show relative risk (comparing risk in two groups), figure four reflects absolute risk (a person's individual risk of developing a disease, over time).
Figure 4 shows the risk of experiencing one of nine adverse events either from COVID-19 illness, vaccination with the J&J COVID-19 vaccine, or vaccination with the two-dose Pfizer COVID-19 vaccine.
- Here, we refer to the nine conditions identified by Barda, 2021 in comparing the absolute risk of COVID-19 infection compared to COVID-19 vaccination.
Our analysis shows that being infected with COVID-19 comes with an equal or significantly higher absolute risk of experiencing an adverse event than having a COVID-19 vaccine.
- Pulmonary embolism is significantly higher (8,871 per 100 000 lives) in people who have COVID-19 than in those who are vaccinated (zero statistical risk) with either vaccine type.
- The risks of acute kidney injury (1,060 per 100 000 lives), arrhythmia (866 per 100 000 lives) and deep-vein thrombosis 512 per 100 000 lives) are also far higher in people who have COVID-19.
- Post-vaccination, we detect no risk (zero statistical risk) of these adverse events.
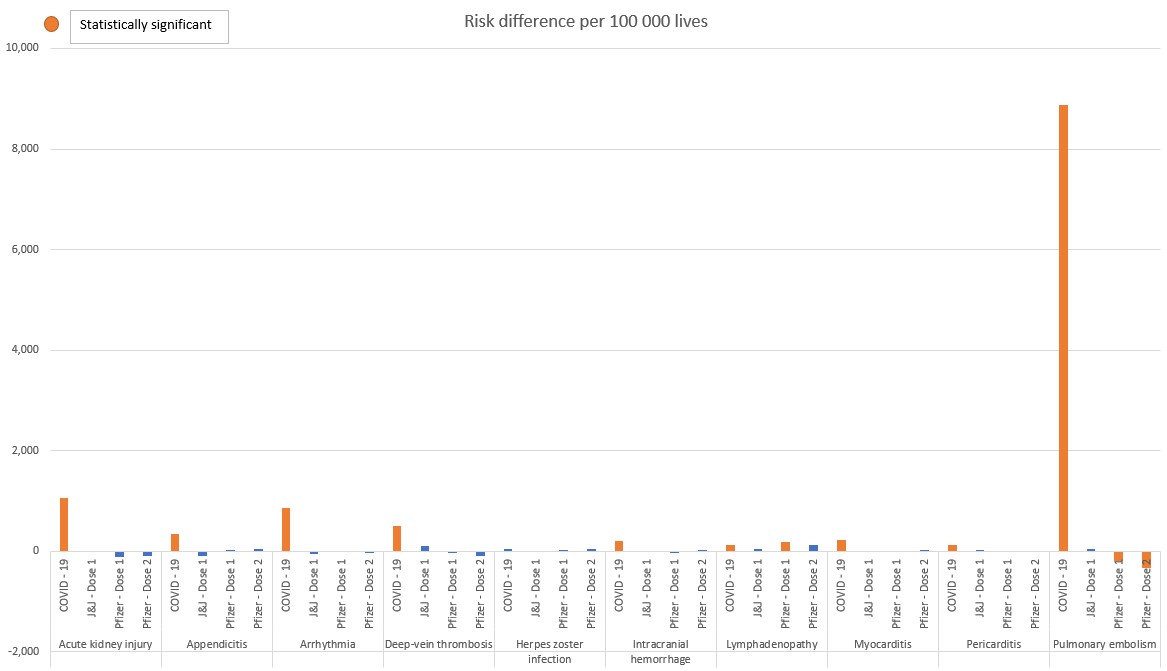
Figure 4: Absolute excess risk of statistically significant adverse events in the case of COVID-19 illness, or COVID-19 vaccination (J&J or Pfizer vaccines)
The risk of three out of 179 conditions was found to be significant in relation to COVID-19 vaccination. However, this risk is outweighed in the case of COVID-19 infection.
Importantly, out of 179 conditions, only 3 identified adverse events identified were linked to COVID-19 vaccination in Figures 1 to 3 (lymphadenopathy, myositis, and paraesthesia), and all are mild in nature as they cause treatable symptoms such as swollen lymph nodes, inflammation in the muscle and numbness.
As shown in Figure 5 the relative risk of experiencing these three adverse events after a single COVID-19 vaccine dose is similar or lower than the risk in the face of COVID-19 disease.
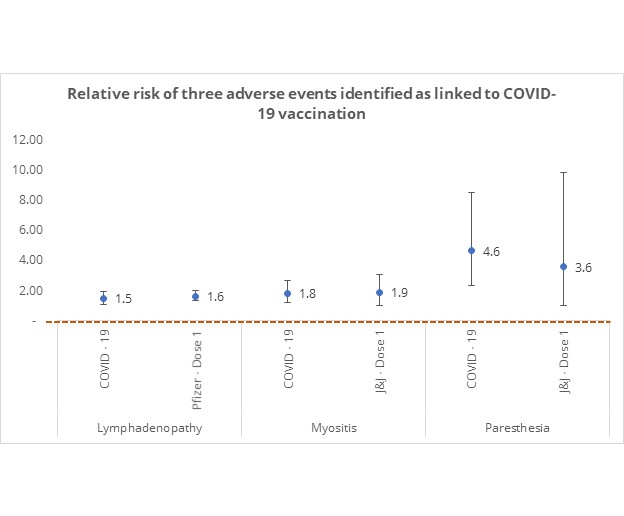
Figure 5: Relative risk of statistically significant adverse events in individuals who have COVID-19 or who have COVID-19 vaccine dose
While the relative risk of these conditions is similar for COVID-19 infection and vaccination, the absolute risk of experiencing these events post-vaccination is far lower than in COVID-19 disease as shown in Figure 6.
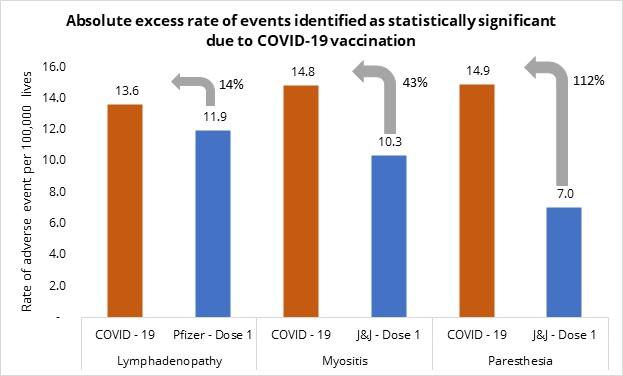
Figure 6: Absolute excess risk of events identified as statistically significant following COVID-19 vaccination, compared in individuals who have COVID-19 or who have had a COVID-19 vaccine dose.
In all cases, COVID-19 poses a higher absolute risk with:
- Paraesthesia occurring in people who have COVID-19 at a 112.10% higher rate than in COVID-19 vaccinated individuals
- Myositis occurring in people who have COVID-19 at a 43.47% higher rate than in COVID-19 vaccinated individuals
- Lymphadenopathy in people who have COVID-19 at a 14.20% higher rate than in COVID-19 vaccinated individuals.
Conclusion
We examined 179 possible conditions which could occur as adverse events post COVID-19 vaccination. Our data show that neither the J&J COVID-19 vaccine nor the Pfizer COVID-19 vaccine is associated with an elevated risk of the vast majority of the 179 adverse events we examined. Here are the major conclusions:
- Out of 179 conditions, only 3 were identified as adverse events linked to COVID-19 vaccination (lymphadenopathy, myositis, and paraesthesia).
- All are mild in nature as they cause treatable symptoms such as swollen lymph nodes, inflammation in the muscle and numbness. Whereas the adverse events linked to COVID-19 infection (pulmonary embolism, myocarditis and acute kidney injury among many others) are more serve in nature.
- The relative risk of these 3 conditions were similar for COVID-19 infection as they were for COVID-19 vaccination (Figure 5)
- The absolute risk of these 3 conditions were higher for COVID-19 infection compared with COVID-19 vaccination (Figure 6)
- Paraesthesia occurred in people who have COVID-19 at a 112% higher rate than in COVID-19 vaccinated individuals
- Myositis occurred in people who have COVID-19 at a 43% higher rate than in COVID-19 vaccinated individuals
- Lymphadenopathy occurred in people who have COVID-19 at a 14% higher rate than in COVID-19 vaccinated individuals. Even though we showed that vaccinated individuals (post-dose one with Pfizer) have a higher risk relative to its matched comparator, the absolute difference is higher for COVID19 infected individuals, as their matched comparators have a higher baseline risk relative to the matched comparators for Pfizer recipients, due to the different demographic profile of vaccinated individuals relative to COVID19 infected individuals.
- For COVID-19 infection:
- There is a 39.6-fold increased risk of pulmonary embolism. This means that people are forty times more likely to experience this condition when they have COVID-19 than when they are not SARS-CoV-2 infected.
- There is a 22.4-fold increased risk of myocarditis.
- There is a 15.78-fold increased risk of acute kidney injury.
Though this analysis does not reflect peer-reviewed data, we believe the results of our robust analysis are highly relevant in that they reflect the experience of almost one million medical scheme members vaccinated between 17 February and 28 September 2021 (approximately seven months' worth of data) and around 175 000 scheme members who had COVID-19 per adverse event considered.
All in all, our findings reinforce the safety of the COVID-19 vaccines being rolled out in South Africa. We make important points in relation to overcoming the misinformation prevalent about the risk of COVID-19 vaccine-related side effects - particularly in relation to the risk of experiencing the same side effects from COVID-19 disease itself.
This data also complements our recently published data on real-world vaccine effectiveness for the Pfizer COVID-19 vaccine in protecting against COVID-19 admission and mortality.
References
1) Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting (Barda et al, September 16 2021, N Engle J Med 2021; 385:1078-1090 Doi: 10.1056/Nejmoa2110475_
2) CDC: People with certain medical conditions
Interested in knowing more or reporting on these findings?
Please contact us on MEDIA_RELATIONS_TEAM@discovery.co.za to request any updated data available since publication and any further context required.
Did you find this post useful?
You may also be interested in reading our related post on Pfizer vaccine real-world effectiveness in which we share our data that the Pfizer vaccine us highly protective against COVID-19 admission and mortality. We also explore the way in which people who have recovered from COVID-19 and are fully vaccinated enjoy significant protection from COVID admission - so called "super immunity" of sorts.
All information shared on this page is based on perspectives gained from analysis of figures and trends emanating from discovery health's data pool. The analysis, which is conducted by discovery health's actuarial and data scientist team, aims to encourage industry dialogue. This content is shared for educational and informational purposes only. It does not constitute peer-reviewed, published scientific research, and hence should not be interpreted as such or used as a basis for altering treatment decisions
Pfizer vaccine's real-world effectiveness in protecting against COVID-19 admission and death in the Discovery Health client population
03 November 2021
Authors: Shirley Collie (Chief Healthcare Analytics Actuary, Discovery Health) and Jared Champion (Senior Actuary, Discovery Health)
Discovery Health's investigation into the Pfizer COVID-19 vaccine's real-world vaccine effectiveness shows a 92% effectiveness in reducing COVID-19 hospital admission risk and 94% effectiveness in preventing the risk of COVID-19 death, between 14 days and 3 months after receiving the second dose.

Super immunity? Discovery Health data analysis shows 98% drop in risk of COVID admission for people who have both recovered from COVID-19 and who are fully vaccinated (Pfizer two-dose vaccine)
03 November 2021
Authors: Shirley Collie (Chief Healthcare Analytics Actuary, Discovery Health) and Jared Champion (Senior Actuary, Discovery Health)
Discovery Health's investigation into the Pfizer COVID-19 vaccine's real-world effectiveness takes a closer look at the impact of full vaccination on those who have recovered from COVID-19. Our investigation shows that for people in this group, the absolute risk of COVID-19 admission is reduced by 98%.