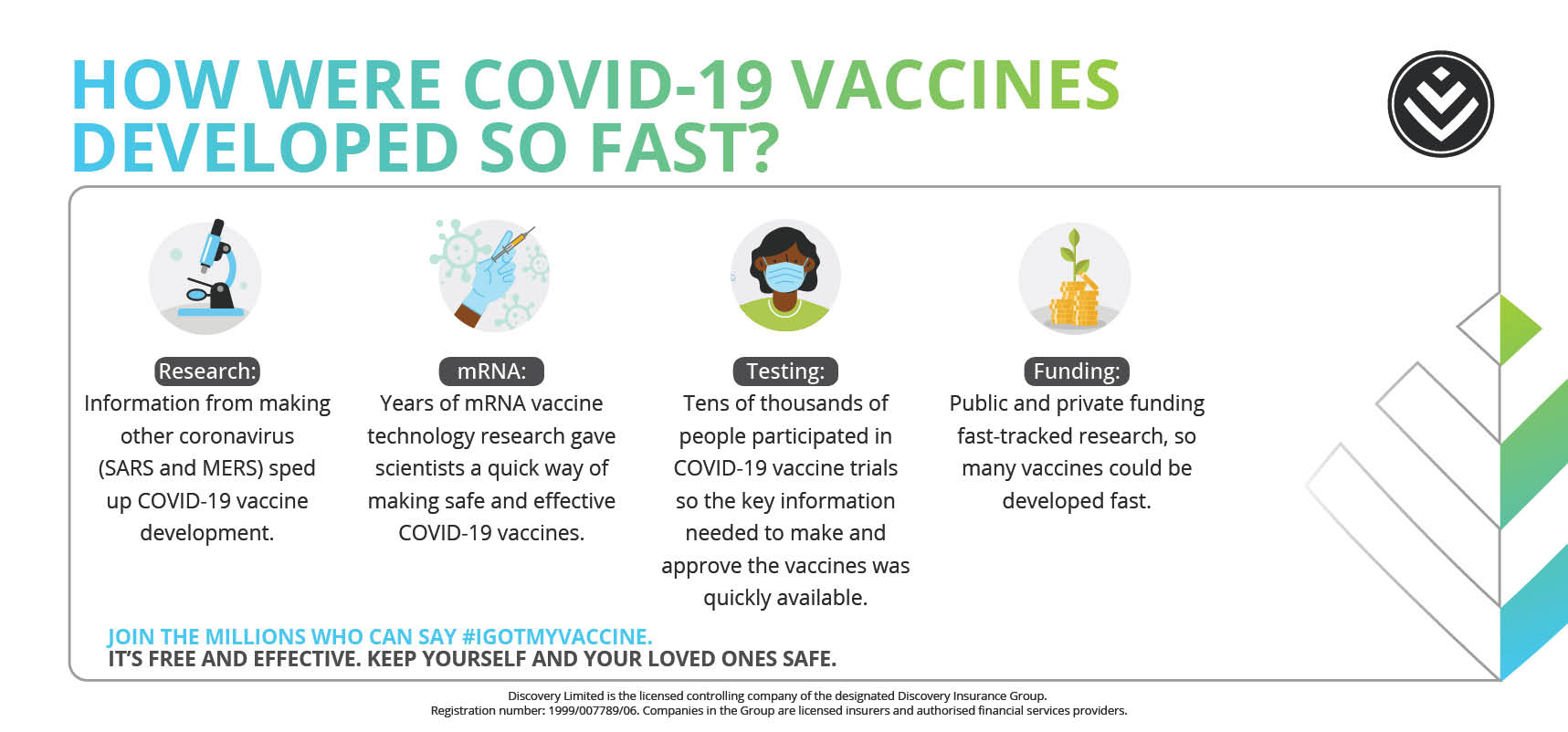
How, and why, were COVID-19 vaccines developed so fast?

Welcome to Video 2 of our series on Understanding Vaccines with Dr Noluthando Nematswerani, Head of the Discovery Health Centre for Clinical Excellence. In this article we learn how COVID-19 vaccines were able to be developed so quickly, and how we can be sure they're still tested enough to be safe and effective.
All across the world, COVID-19 is a public health emergency. Over 4 million people have lost their lives globally to the disease. Millions of others have been infected, sometimes becoming seriously ill for days, weeks or even months. Healthcare systems worldwide have been severely strained since the start of the pandemic.
As a result, it was a top priority globally for vaccine developers to use existing technologies to our advantage so they could fast-track the development of COVID-19 vaccines. This was to save lives, and to relieve the burden on healthcare systems.
The rigorous stages of vaccine development
Vaccines generally take many years to develop before they become commercially available, and the progress is highly dependent on the resources and findings available.
The vaccine development process is made up of a number of stages. Testing starts in the lab. Once it works there, you test the vaccine on animals, and only once it works there can you do clinical trials on humans. Human trials take three stages before vaccines can be authorised for use.
- Phase 1 involves small studies where you're testing safety and the appropriate dosing.
- Phase 2 involves hundreds of patients, where you test again for safety and efficacy.
- Phase 3 involves multi-centre trials, which are randomised controlled trials where you test once more for efficacy before you can register the vaccine for use more broadly.
- After Phase 3 trials of testing, there is a process of submission and approval that's required before a vaccine can be distributed and administered to the public.
All the COVID-19 vaccines available have been tested and authorised for use.
The one good thing about COVID-19 being a worldwide problem was that scientific expertise and resources from all around the globe worked together to ensure quicker access to vaccines.. Lots of funding was secured, which meant more could get done efficiently. And the testing and production phases were done in parallel, to speed up the process. This was so people everywhere would have access to life-saving vaccines as quickly as possible. Watch Dr Noluthando explain it here:
It's important to know that all the COVID-19 vaccines that have currently been authorised have passed every testing stage. They were granted emergency-use authorisation, for the ongoing emergency that is the COVID-19 pandemic.
Having safe, effective COVID-19 vaccines available is a global triumph!
So embrace the triumph of human cooperation by understanding the true feat of having safe, effective COVID-19 vaccines available to you today! Learn more about COVID-19 and vaccines here, and join the millions of South Africans who can proudly say, #IGotMyVaccine.