Post COVID series (2 of 3): Does getting COVID-19 increase your risk of death in the immediate and long-term?

For some, recovery from COVID-19 comes with prolonged, persistent, related symptoms and complications. In this - the second of three articles in this series - we share our analysis of long-term mortality outcomes post COVID-19 recovery.
Authors: Discovery Health's Shirley Collie (Chief Healthcare Analytics Actuary), Lebohang Radebe (Data Scientist), Tommy Chen (Actuary), Jared Champion (Senior Actuary), Donald Ntjana (Senior Data Scientist), Dr Dave Jacobs (Senior Clinician and Clinical Classification System Architect), Dr Smybinn Mathews (Clinical Risk Specialist), Sameera Haneef (Senior Actuary), Lizelle Steenkamp (Senior Healthcare Statistician) and Matt Zylstra (Senior Actuary)
A three-part analysis and article series
- This is the second of three articles assessing Discovery Health administered medical scheme members' long-term experience, post COVID-19 recovery. Here, we cover mortality trends. We look at death rates in the months after medical scheme members included in our study recovered from COVID-19.
- In our first article in the series we provided feedback on healthcare utilization trends - specifically, we measured admission rates in different groups - again, post recovery from COVID-19.
- And, in the third article in this series, we provide feedback on the risk of onset of new chronic conditions after recovery from COVID-19.
Background to our analyses
By 31 May 2022, 18.5% (609,890 members) of Discovery Health administered medical scheme members had experienced a documented COVID-19 infection.
The immediate clinical and financial impact of acute COVID-19 has been well-described in numerous global publications [1,2]. What the local and global scientific community is still documenting is the detail around long-term healthcare utilisation patterns and mortality impact in people who recover from COVID-19. In other words, once people get and recover from COVID-19, how likely are they to experience other health-related events in the future that are related to their having got COVID-19?
- It is important to note that our findings very likely offer a conservative perspective given the likelihood of a high number of undocumented COVID-19 infections in the control, reference population.
Our methodology
For the purposes of this analysis, we considered Discovery Health administered medical scheme member data for the period 1 March 2020 - 30 November 2021.
- 404,067 members were included in the analysis (these individuals had a documented COVID-19 infection determined by a positive PCR test or a healthcare provider's diagnosis).
1) Establishing a test and a control group
We applied a bootstrapping technique to this study cohort in order gain insight into the statistical significance of our findings. Specifically, we randomly selected 404,067 individuals with replacement 500 times to create bootstrap samples of members with an equivalent size to our study cohort size. For each individual who had COVID-19 (test group member), we randomly selected a maximum of 100 potential comparators (clinical comparator) who'd had no documented COVID-19 infection but who had the same underlying health risk factors at two and three months prior to the test group member's COVID-19 infection. We then randomly matched each infected individual to a clinical comparator within each bootstrap without replacement (i.e., there was no duplication of control comparators within a bootstrap).
We refer to these pairs of matched infected and uninfected individuals as clinical twins.
2) Why did we look at the period two and three months prior to documented infection date in carrying out our matching?
We went back three months prior to infection for matching, as in the first month prior to the documented infection date, we noted increased healthcare utilisation for the COVID-19 infected individual. Given that the date of documented infection may be several days after the actual clinical infection date, this utilisation was likely attributable to infection, and we wanted to match on patient characteristics independent of utilisation attributable to the acute infection phase. We then specifically selected two time periods - two and three months prior to documented infection date - to ensure that the trend of healthcare utilization patterns in the period prior to infection was consistent between the study population and their matched clinical twins.
This method enabled us to construct a statistical confidence interval around our uninfected population which incorporated both the variability in the demographics of the underlying population being evaluated and the consequent variability of outcomes.
3) The underlying health risk factors we matched on included:
- Age : <1; 1-4; 5-11; 12-18; 19-64 (clinical twins were matched within one year of age of each other); 65+
- Member's sex : Male (M) or Female (F)
- Location : Province in South Africa
- Number of chronic conditions : 0, 1, 2 or more
- Number of prior flu vaccinations : 0, 1, 2 or more. People who had a flu vaccination in the years leading up to and during the COVID-19 pandemic were likely to be more aware of promoting their general health and wellbeing and adhere to preventive measures.
- One of five medical scheme product options : Looking at out-of-hospital benefits we divided the 24 plans available through both Discovery Health Medical Scheme and 18 of the Discovery Health administered schemes, into 5 groups.
- Vaccination status : Having had at least one dose of any COVID-19 vaccine prior to a member's first COVID-19 infection; or unvaccinated
- Diabetes status: Registered on a Chronic Care Benefit (CCB) for this disease; or not registered.
- Hypertension status : Registered on a CCB for this disease; or not registered.
- Ischaemic heart disease status : Registered on a CCB for this disease; or not registered.
- Number of in-hospital admissions: In the two and three months prior to COVID-19 infection.
- Probability of an in-hospital admission within the next six months (matched within 2.5% of each other). To work out the probability of an in-hospital admission we used a separate predictive model, which took the following factors into account:
- Chronic conditions a member was registered for
- Demographic factors such as age, sex and type of medical scheme plan the member was on
- The sort of doctors the member had seen in the past 12 months
- Medicines for a member had claimed for in the past 12 months
- Procedures and consultations a member had claimed for in the past 12 months
- ICD10 information from claims submitted in the past 12 months
We compared clinical twins' experiences from the three months before the test group member got COVID-19 and for up to 12 months after the test group member recovered.
- The test group member's recovery date from COVID-19 was based on either 14-days post a test group member's positive PCR collection date or, where they were admitted to hospital, their discharge date.
4) Matched clinical twin pairs were removed from the analysis from the point of time in the follow up period when:
- Either person withdrew their membership of a Discovery Health administered medical scheme
- Either person died
- The person who got COVID-19 was vaccinated after their infection, or the matched clinical twin was vaccinated (so as not to confound the effects on clinical outcomes).
- The matched clinical twin had a documented COVID-19 infection
- The person who got COVID-19 (test group) had a second documented COVID-19 infection.
5) Deaths were assessed over two periods - in the COVID-19 acute phase (when a person had COVID-19) and recovery phase (the 12 months that followed). What did we measure?
Given that the actual number of deaths vary by member demographics (i.e., by sex, age, number of chronic conditions, and so on), we standardised our analysis by examining the number of deaths per "1,000 life months". This measure looks at the number of deaths per 1,000 members, per month and allows us to compare our results across the different sub-cohorts. Given that the duration of period zero is not standardized at a month (the time frame ranges from a minimum of two weeks for non-admitted patients, to a maximum two weeks after discharge for admitted patients), we refer to the number of deaths per 1,000 infections during the acute period of infection (period 0).
We looked at:
Mortality risk: How much higher is the risk of dying once people have had and recovered from COVID-19, compared to a matched clinical twin (who didn't get COVD-19)? We worked this out as:
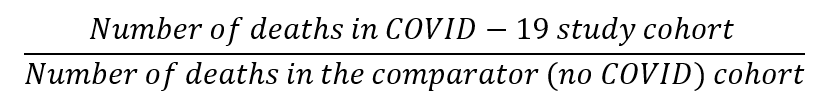
A result, or ratio >1 means that in the months after people get and recover from COVID-19, there's a higher mortality rate in the COVID-19 study population relative to the non-COVID19 population. Where the relative risk ratio is <1, the opposite is true.
Definitions:
Number of deaths : The absolute number of deaths in the group that got COVID-19 per 1,000 infections or life months, over a particular period
Excess deaths : The number of deaths in the COVID-19 population that were over and above the number of deaths in the non-COVID-19 population, per 1,000 infections or life months. The higher the number of excess deaths, the more that people in the COVID-19 population died in the period of infection and up to twelve months thereafter, than did the general population.
We worked this out as :
Deaths in the COVID-19 test group - Deaths in the comparator group
We assessed the statistical significance of our findings to confirm that the differences we saw were unlikely to be due to underlying population variability alone.
Excess deaths were compared against those of their matched clinical twin. If the 95% confidence interval of the difference in outcomes did not cross zero (i.e., no difference observed), we deemed them to have a statistically significant higher outcome rate.
In the time after recovery from COVID-19, the average mortality risk,average number of deaths and average excess deaths were weighted by the total exposure (the changing number of matched clinical twins over time) in order to account for the decreasing number of clinical twins under observation over time.
6) What types of deaths did we look at?
Overall, we looked at mortality, both related and unrelated to COVID-19. These deaths were further assessed by looking at:
- Member's sex : Male (M) or Female (F)
- Number of chronic conditions : none, 1 to 2, or 3
- Ages : Brackets looked at were <1, 1-4, 5-11, 12-18, 19-39, 40-49, 50-59, 60-69, 70-79 and 80+
- Likely COVID-19 variant contracted , based on when people got COVID-19 and which wave of infection the country was in at the time. Variant options were D614G (wave 1), Beta (wave 2) or Delta (Wave 3).
- Vaccination status : Unvaccinated or fully vaccinated. Fully vaccinated individuals were those who'd had a COVID-19 infection at least 14 days after dose two of any COVID-19 vaccine. South Africa's first phase of COVID-19 vaccination (17 February to 15 April) focused on healthcare workers. It was on 16 April that vaccination opened up to essential workers and the elderly. Most members of the public only became eligible for and accessed COVID-19 vaccines later in the year, so we have only five months of follow-up data for medical scheme members who were fully vaccinated before their first COVID-19 infection.
- How serious any admission was : Members were assigned to a group according to the most serious phase of their admission. These included Ventilation (considered most serious or 'highest acuity'), care in ICU, High Care or a General ward or 'not hospitalized'.
Findings: Match quality and number of clinical twin observations over time
Overall, we were able to find potential twin matches for 88.8% (n = 358,887) of our COVID-19 test group cohort.
Younger, healthier people had a bigger chance of a potential match. After completing the matching process, our analysis included 80.2% of medical admissions, 77.3% of deaths recorded, and 86.2% of people aged 65 and over (Table 1).
|
Cohort |
Total number of clients |
Matched |
Average % of total medical admissions included in our cohort after matching |
Average % of deaths included our cohort after matching |
Average % of people aged 65 and over, who were matched |
|
|
Average number of clients across 500 bootstraps |
% of total |
% |
% |
% |
||
|
Overall |
404,067 |
358,887 |
88.8 |
80.2 |
77.3 |
86.2 |
|
Sex |
||||||
|
Female |
216,659 |
223,715 |
88.0 |
80.2 |
78.3 |
88.0 |
|
Male |
185,696 |
190,312 |
88.2 |
80.6 |
76.9 |
84.3 |
|
Number of Chronic Conditions |
||||||
|
None |
255,528 |
243,216 |
95.2 |
94.0 |
92.2 |
93.2 |
|
One to Two |
105,700 |
84,456 |
79.9 |
76.7 |
78.1 |
88.4 |
|
Three or More |
43,469 |
29,038 |
66.8 |
66.6 |
69.5 |
82.2 |
|
Age bands |
||||||
|
<1 |
303 |
281 |
92.8 |
89.0 |
||
|
1-4 |
4,777 |
4,600 |
96.3 |
94.8 |
58.5 |
|
|
5-11 |
12,570 |
12,249 |
97.5 |
90.7 |
100.0 |
|
|
12-18 |
24,463 |
23,697 |
96.9 |
89.9 |
75.5 |
|
|
19-39 |
157,346 |
142,645 |
90.7 |
84.6 |
79.8 |
|
|
40-49 |
85,972 |
75,353 |
87.7 |
81.3 |
76.6 |
|
|
50-59 |
60,810 |
49,627 |
81.6 |
76.3 |
71.3 |
|
|
60-69 |
32,474 |
25,897 |
79.8 |
75.9 |
73.5 |
|
|
70-79 |
17,186 |
14,782 |
86.0 |
83.4 |
81.8 |
|
|
80+ |
7,552 |
6,482 |
85.8 |
84.2 |
84.1 |
|
|
Circulating COVID-19 Variant |
||||||
|
D614G |
86,825 |
77,648 |
89.4 |
84.2 |
84.8 |
90.0 |
|
Beta |
104,089 |
86,734 |
83.3 |
75.6 |
70.3 |
81.4 |
|
Delta |
214,852 |
193,267 |
90.0 |
82.5 |
80.2 |
87.9 |
|
Vaccination status |
||||||
|
Un-vaccinated |
367,487 |
323,615 |
88.1 |
80.4 |
77.1 |
85.4 |
|
Fully vaccinated |
3,982 |
3,568 |
89.6 |
82.8 |
80.0 |
90.0 |
|
Highest acuity |
||||||
|
Hospitalized: Ventilation |
5,693 |
4,546 |
79.9 |
79.9 |
79.9 |
85.9 |
|
Hospitalized: ICU |
8,729 |
6,852 |
78.5 |
78.7 |
77.0 |
83.3 |
|
Hospitalized: High Care |
9,014 |
7,318 |
81.2 |
81.3 |
78.4 |
85.8 |
|
Hospitalized: General Ward |
46,255 |
37,666 |
81.4 |
80.8 |
75.5 |
83.7 |
|
Not hospitalized |
344,243 |
306,825 |
89.1 |
77.4 |
79.5 |
88.2 |
Table 1. Categories considered in our analysis of Discovery Health administered medical scheme members who had a documented COVID-19 infection and who were matched to a clinical twin (1 March 2020 - 30 November 2021).
An overall 88.8% match rate resulted in a mean of 358,887 [95% CI: 358259, 359516] members per bootstrap in the infection period. Keep in mind that we removed clinical twins from our analysis where there were scheme withdrawals, deaths, new infections, re-infections, or COVID-19 vaccinations in the post-infection or follow up period.
In the follow-up period, we experienced a steady decrease in the number of members under observation - from a high of 305,535 [95% CI: 304870, 306202] members in month one since recovery to 33,512 [95% CI: 33178, 33846] members at 12 months post recovery (Figure 1).
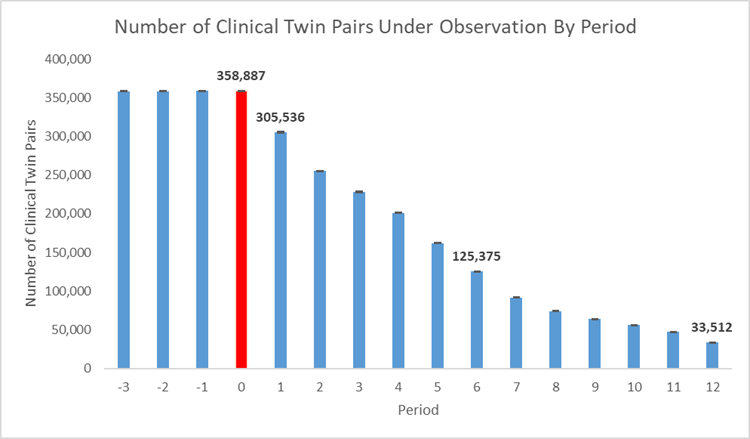
Figure 1. Number of clinical twins under observation over time where red (period 0) shows the acute infection period.
Findings: Overall mortality rates are highest in the month when people get COVID-19
While people are infected with COVID-19 (our 'infection period' or period 0 seen in Figures 2a and b) mortality rates increased significantly compared to matched clinical twins who didn't get COVID-19. Then, in what we consider the 'recovery phase' in the one to twelve months after their infection we don't find any evidence of increased mortality rates, again compared to matched clinical twins who didn't get COVID-19.
Specifically, we see (Figure 2a):
- A 75 times higher mortality risk during the infection phase
- An average of 23.0 deaths per 1,000 infections during the infection period
- No evidence of mortality in the 12 months that follow, or the 'recovery phase'
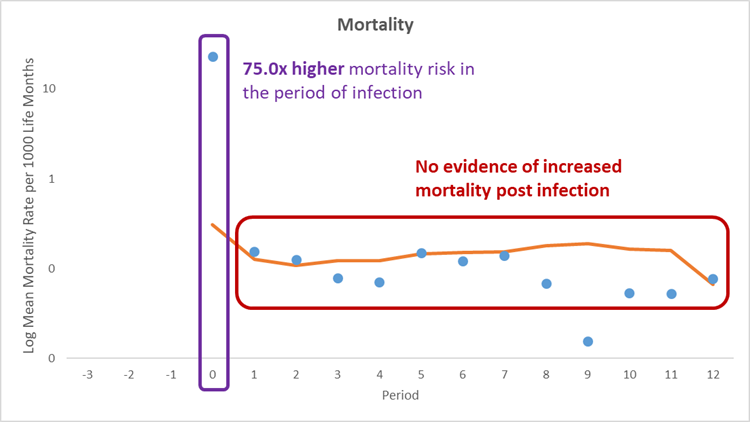
Figure 2a) All-cause mortality rate per 1,000 life months for the COVID-19 population and comparator population over time. Blue dots show the mean of COVID-19 infected members. Orange line shows the mean of uninfected individuals.
The increased mortality risk seen in the infection phase (which we call 'excess utilisation') resulted in (Figure 2b):
- 22.7 additional deaths per 1,000 infections in the period of infection
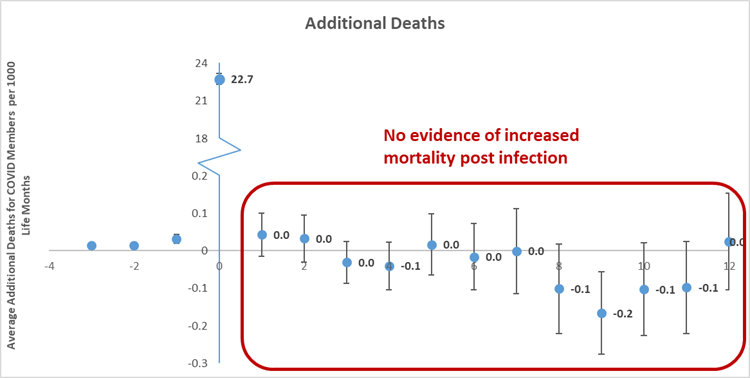
Figure 2b) Average number of additional all-cause deaths for COVID-19 members per 1,000 life months
With the above in mind, we conducted further analysis of our clinical twin sets to extract insights into long-term mortality risk within various sub-groups (cohorts).
What mortality-related differences do we see in men and women?
Overall, we see that during the period of infection, men with documented COVID-19 infection experience a higher relative and absolute mortality risk against their matched clinical twins compared to women with documented COVID-19 infection against their matched clinical twins. Males experience 33 excess deaths whilst females experience 20 excess deaths per 1,000 infections. In the post recovery period, there is no evidence of increased mortality. (Table 2)
|
Sex |
Period of Infection |
Post recovery |
|||||
|
Mortality risk |
Number of deaths per 1,000 infections for the COVID-19 population |
Number of excess deaths per 1,000 infections |
Number of significant months (NSM) |
Weighted average times higher mortality risk until NSM |
Weighted average number of deaths per 1,000 life months until NSM for COVID-19 population |
Weighted average additional deaths per 1,000 life months in each of the NSM |
|
|
Female |
71.1 |
20.4 |
20.1 |
- |
- |
- |
- |
|
Male |
78.4 |
33.7 |
33.3 |
- |
- |
- |
- |
Table 2. Summary of mortality risk by members' sex. Highlighted areas indicate the highest risk by sex.
What is the impact of living with chronic conditions on mortality risk post COVID-19 recovery?
Overall, we see that during the period of infection, members with no chronic conditions experience the highest relative mortality risk, whereas members with three or more chronic conditions experience the highest excess absolute mortality risk- those with documented COVID-19 infection and no chronic conditions experience 128 times higher risk and 11 excess deaths per 1,000 infections whereas those with documented infection and three or more chronic conditions experience 58 times higher mortality risk and 126 excess deaths per 1,000 infections. In the post recovery period, there is no evidence of increased mortality. (Table 3)
|
Number of Chronic Conditions |
Period of Infection |
Post recovery |
|||||
|
Mortality risk ratio (mortality risk of infected vs uninfected clinical twins) |
Number of deaths per 1,000 infections for the COVID-19 population |
Number of excess deaths per 1,000 infections |
Number of significant months (NSM) post recovery |
Weighted average times higher mortality risk until NSM |
Weighted average number of deaths per 1,000 life months until NSM for COVID-19 population |
Weighted average additional deaths per 1,000 life months in each of the NSM |
|
|
None |
128.0 |
11.1 |
11.0 |
- |
- |
- |
- |
|
One to Two |
80.9 |
35.9 |
35.5 |
- |
- |
- |
- |
|
Three or More |
58.0 |
128.5 |
126.3 |
- |
- |
- |
- |
Table 3. Summary of mortality risk by members' chronic condition count. Highlighted areas indicate the highest risk by number of chronic conditions.
What's the impact of age on mortality risk?
We see that during the period of infection, younger members experience lower excess mortality risk, whereas older members experience higher absolute excess mortality risk. In the post recovery period, there is no evidence of increased mortality across all age groups (Table 4).
|
Age Group |
Period of Infection |
Post recovery |
|||||
|
Mortality risk ratio (infected vs unifected) |
Number of deaths per 1,000 infections for the COVID-19 population |
Number of excess deaths per 1,000 infections |
Number of significant months (NSM) |
Weighted average times higher mortality risk until NSM |
Weighted average number of deaths per 1,000 life months until NSM for COVID-19 population |
Weighted average additional deaths per 1,000 life months in each of the NSM |
|
|
<1 |
- |
- |
- |
- |
- |
- |
- |
|
1-4 |
2.0 |
0.4 |
0.4 |
- |
- |
- |
- |
|
5-11 |
3.3 |
0.2 |
0.2 |
- |
- |
- |
- |
|
12-18 |
10.8 |
0.4 |
0.4 |
- |
- |
- |
- |
|
19-39 |
132.3 |
4.1 |
4.0 |
- |
- |
- |
- |
|
40-49 |
116.8 |
15.2 |
15.0 |
- |
- |
- |
- |
|
50-59 |
125.3 |
34.4 |
34.0 |
- |
- |
- |
- |
|
60-69 |
72.2 |
73.7 |
72.6 |
- |
- |
- |
- |
|
70-79 |
53.5 |
153.1 |
150.2 |
- |
- |
- |
- |
|
80+ |
82.5 |
290.7 |
287.0 |
- |
- |
- |
- |
Table 4. Summary of mortality risk by members' age. Highlighted areas indicate the highest risk by age.
What is the impact of the COVID-19 variant contracted on mortality risk?
Overall, we see that during the period of infection, members with documented infection in South Africa's Beta driven wave experienced the highest number of excess deaths per 1000 infections - 35, whereas those infected in South Africa's Delta driven wave experienced the lowest number of excess deaths per 1,000 infections - 23. Vaccination became available to Discovery clients well after the peak of the Beta wave, and the difference in vaccination rates between waves is likely to have contributed to the observed differences in the acute period. Whilst those infected in the Delta wave were found to have a statistically significant higher mortality risk one month post recovery, the quantum of this is small- 0.1 per 1000 life months. In the post recovery period months 2-12, there is no evidence of increased mortality regardless of period of documented infection (Table 5).
|
Circulating COVID-19 Variant |
Period of Infection |
Post recovery |
|||||
|
Mortality risk |
Number of deaths per 1,000 infections for the COVID-19 population |
Number of excess deaths per 1,000 infections |
Number of significant months (NSM) |
Weighted average times higher mortality risk until NSM |
Weighted average number of deaths per 1,000 life months until NSM for COVID-19 population |
Weighted average additional deaths per 1,000 life months in each of the NSM |
|
|
D614G |
61.2 |
27.6 |
27.1 |
- |
- |
- |
- |
|
Beta |
102.8 |
35.1 |
34.7 |
- |
- |
- |
- |
|
Delta |
72.8 |
22.8 |
22.5 |
2 |
7.4 |
0.1 |
0.1 |
Table 5. Summary of mortality risk by members' COVID-19 variant. Highlighted areas indicate the highest risk by circulating COVID-19 variant.
What is the impact of vaccination status on mortality risk?
Overall, we see that during the period of infection, unvaccinated members experienced the highest number of excess deaths per 1,000 infections - 27, whereas those fully vaccinated experienced the lowest number of excess deaths per 1,000 infections - 6. In the post recovery period, there is no evidence of increased mortality regardless of vaccination status (Table 6).
|
Circulating COVID-19 Variant |
Period of Infection |
Post recovery |
|||||
|
Mortality risk |
Number of deaths per 1,000 infections for the COVID-19 population |
Number of excess deaths per 1,000 infections |
Number of significant months (NSM) |
Weighted average times higher mortality risk until NSM |
Weighted average number of deaths per 1,000 life months until NSM for COVID-19 population |
Weighted average additional deaths per 1,000 life months in each of the NSM |
|
|
Un-vaccinated |
73.1 |
27.0 |
26.6 |
- |
- |
- |
- |
|
Fully vaccinated |
-* |
5.7 |
5.7 |
- |
- |
- |
- |
Table 6. Summary of mortality risk by members' vaccination status. Highlighted areas indicate the highest risk by vaccination status.
*0 deaths observed in the comparator
What is the impact of acuity of admission on post recovery mortality risk?
Overall, members who were ventilated during their period of infection experience the highest relative and absolute increase in mortality risk in the period of infection - 584 times higher mortality risk, translating to 643 additional deaths per 1,000 infections (Table 7). Members with documented infection, that did not require admission to hospital experienced the lowest additional deaths per 1000 infections during the period of infection.
|
Highest Acuity |
Period of Infection |
Post recovery |
|||||
|
Mortality risk |
Number of deaths per 1,000 infections for the COVID-19 population |
Number of excess deaths per 1,000 infections |
Number of significant months (NSM) |
Weighted average times higher mortality risk until NSM |
Weighted average number of deaths per 1,000 life months until NSM for COVID-19 population |
Weighted average additional deaths per 1,000 life months in each of the NSM |
|
|
Hospitalized: Ventilation |
583.6 |
644.6 |
643.3 |
- |
- |
- |
- |
|
Hospitalized: ICU |
258.5 |
468.0 |
446.0 |
- |
- |
- |
- |
|
Hospitalized: High Care |
56.0 |
112.6 |
110.4 |
- |
- |
- |
- |
|
Hospitalized: General Ward |
53.0 |
65.9 |
64.6 |
- |
- |
- |
- |
|
Not Hospitalized |
17.9 |
3.1 |
2.9 |
- |
- |
- |
- |
Table 7. Summary of mortality risk by members' highest acuity during their period of infection. Red indicates the highest risk by highest acuity.
Conclusion
In this, the second of our three-part post-COVID impact analysis series, we have considered the increased risk of death during the period of infection with COVID-19 and up to 12 months after.
We look at this risk according to:
- Sex
- Number of chronic conditions
- Age
- Likely COVID-19 variant contracted
- Vaccination status
- Highest acuity of admission during period of COVID-19 infection
In summary, we found that:
- A generally increased (75 times higher on average) mortality risk during the acute COVID-19 infection period.
When they got COVID-19, we saw an increased risk of death for:
- Men (78 times higher, with 33 excess deaths per 1,000 infections)
- Women (71 times higher, with 20 excess deaths per 1,000 infections)
- People with no chronic illnesses (128 higher risk of death) and, people with three or more chronic illnesses or co-morbidities (58 times higher risk of death).
- We also saw higher excess death rates per 1,000 infections in people with three or more chronic illnesses at 126 deaths per 1000 infections compared to only 11 excess deaths in people who had no chronic illnesses.
- Younger people (ages 19 to 39): A 132 times higher risk of death with 4 excess deaths per 1,000 infections
- Middle-aged people (ages 50 to 59): A 125 times higher risk of death with 34 excess deaths per 1,000 infections
- Older people (ages 70 to 79): A 54 times higher risk of death with 150 excess deaths per 1,000 infections
What happened in the year that followed their infection?
- Encouragingly, once study participants recovered from COVID-19, there was no evidence of increased risk of mortality in the months that followed. This was true regardless of member sex, age or comorbidity profile.
How did the COVID-19 variant people contracted affect their risk of death?
- Risk of death during the infection phase was highest during the Beta wave (103 times increased risk of death with 35 excess deaths per 1,000 infections), followed by the Delta wave (73 times increased risk of death with 23 excess deaths), and lowest in the D614G wave (61 times increased risk of death with 27 excess deaths). In the case of the Delta variant, we also saw a 7.4 times higher risk of death in month two post recovery from COVID19 infection, however this translates to only 0.1 excess deaths per 1,000 infections.
How did being fully vaccinated affect these outcomes?
- Fully vaccinated members who got COVID-19 experienced 6 excess deaths per 1,000 infections while infected
- While, in those who were unvaccinated when they got COVID-19, there was much higher (73-fold) risk of death while infected and we recorded 27 excess deaths per 1000 infections in this group during the infection phase.
How did people's highest level of admission acuity during their COVID-19 period of infection affect their risk of death?
- Both relative and absolute risk of death during the infection phase was highest in members who were ventilated during their period of infection (584 times increased risk of death with 643 excess deaths per 1,000 infections) and was lowest in members not hospitalized during their acute period of infection (18 times increased risk of death with 3 excess deaths per 1,000 infections).
Understanding heightened risk of death when infected with COVID-19 versus deaths and excess deaths recorded
In general, our data show that during the acute infection period the highest increase in relative risk of death to COVID-19 is in healthier people (people aged 19 to 39 and those with no chronic illnesses) who are generally at lowest risk of severe COVID-19 outcomes. This is because while some younger and healthier people may die of COVID-19, the death rate of their matched clinical twins is relatively low as few young, healthy people die of non-COVID-19 related causes in the general population. Conversely, while a greater number of older, sicker people may die of COVID-19, the death rate of their matched clinical twins is relatively high as more older, sicker people die of non-COVID-19 related causes in the general population. Therefore, younger and healthier people who die of COVID-19 in the period of infection seemingly have the highest increase in their relative risk of death.
However excess deaths - the number of people who actually die - are highest for those considered at highest risk of serious COVID-19 outcomes - people with multiple chronic illnesses, given the higher absolute underlying risk in this population.
Our third article in this series looks at the risk of developing a chronic illness in the months post COVID-19 infection, adding to the picture we are building around the immediate and long-term impacts of contracting this disease.
- Authors of this article note that the above findings are representative of our medical scheme member population mix, and the long-term patterns observed may be different in other populations and in different clinical settings. It is also important to note this data analysis considers claims data (i.e., claims submitted for members of medical schemes administered by Discovery Health).
Acknowledgements
We would like to thank Discovery Health's Business Intelligence and Big Data Administration teams for the data assets and substantial computational power made available to facilitate our work on this study, as well as Discovery Health's Clinical Coding Intelligence team.
References
- Graves JA, Baig K, Buntin M. The Financial Effects and Consequences of COVID-19: A Gathering Storm. JAMA. 2021;326(19):1909-1910. doi:10.1001/jama.2021.18863
- Bang JY, Hawes R, Varadarajulu S. Clinical, financial and academic implications of COVID-19 on a tertiary care interventional endoscopy programme. Gut 2021; 70:1431-1434.
- Kaye AD, Okeagu CN, Pham AD, et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: International perspectives. Best Pract Res Clin Anaesthesiol. 2021;35(3):293-306. doi: 10.1016/j.bpa.2020.11.009
- Yan Xie, Evan Xu, Benjamin Bowe and Ziyad Al-Aly Long-term cardiovascular outcomes of COVID-19. Nature Medicine volume 28, pages583-590 (2022)
Interested in knowing more or reporting on these findings?
Please contact us on MEDIA_RELATIONS_TEAM@discovery.co.za to request any updated data available since publication and to obtain any further context required.
Did you find this post useful?
Read our related posts, the first and third article in this three-part series in which we consider post-COVID risk of admission to hospital and chronic illness registration - with analysis for three months prior to and up to 12 months post infection.
Read our related post (not part of this three-part Long COVID utilisation series) titled "Long COVID symptoms, impact on carrying out daily activities: Survey of just over 7,000 medical scheme members reveals key insights." We find that an alarmingly high number of medical scheme members have protracted COVID-19 symptoms (long COVID), often with disruption to daily living. The risk of these events is higher in women, in those admitted to hospital or ICU for COVID-19, and those with high morbidity and pre-existing chronic illness. Being COVID-19 vaccinated and regular physical activity lower this risk.
Disclaimer
All information shared on this page is based on perspectives gained from analysing data acquired by Discovery Ltd and its various affiliate entities (Discovery). The analysis, which is conducted by Discovery's actuarial and data science team, aims to encourage industry dialogue. Publications containing our analyses are shared for educational and informational purposes only. Each publication reflects only the data available for analysis at the time of publication. It does not, unless otherwise indicated, constitute peer-reviewed, published scientific research, and hence should not be interpreted as such or used as a basis for altering treatment decisions. While every effort has been made to ensure the accuracy of the content conveyed, we cannot be held liable or responsible for any actions or decisions taken based on the information shared in this article.

Deep dive: Admission rates across South Africa's four COVID-19 waves confirm Omicron-driven fourth wave's lower severity, and more.
07 July 2022
Authors: Chana Suttner (Actuary, Discovery Health),Michael Cohen (Actuarial Analyst, Discovery Health), Shirley Collie (Chief Healthcare Analytics Actuary, Discovery Health

Long COVID symptoms, impact on carrying out daily activities: Survey of just over 7,000 medical scheme members reveals key insights
16 June 2022
Authors: Shirley Collie (Chief Healthcare Analytics Actuary, Discovery Health), Lizelle Steenkamp (Senior Healthcare Statistician, Discovery Health), Lebohang Radebe (Data Scientist, Discovery Health), Dr Smybinn Mathews (Clinician, Discovery Health) and Dr Dave Jacobs (Senior Clinician and Clinical Classification System Architect, Discovery Health)

Discovery's COVID-19 personal resilience index predicts an individual's resilience to serious COVID-19 illness
23 May 2022
Authors: Lizelle Steenkamp (Senior Healthcare Statistician at Discovery Health), Tommy Chen (Actuary at Discovery Health), Jared Champion (Senior Actuary at Discovery Health) and Donald Ntjana (Senior Data Scientist at Discovery Health)